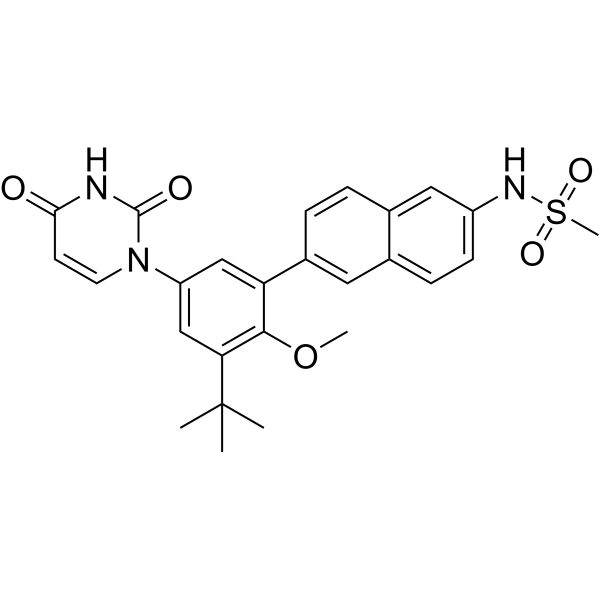
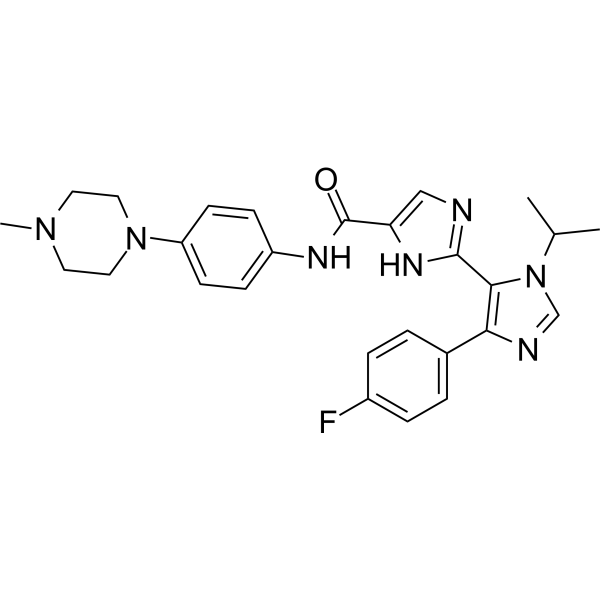
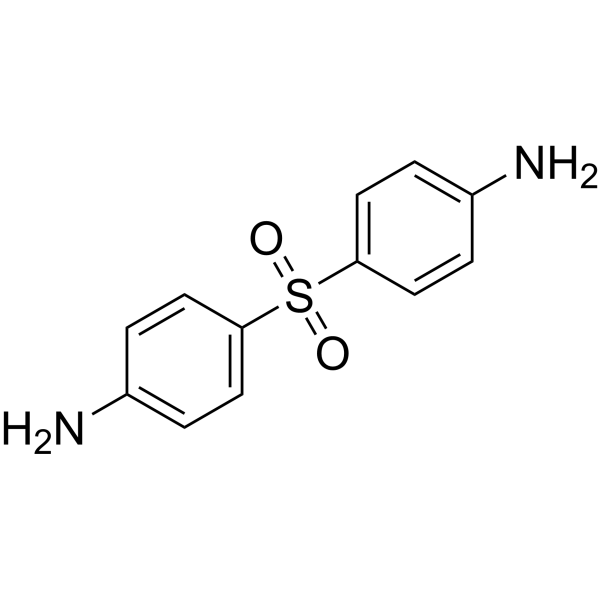
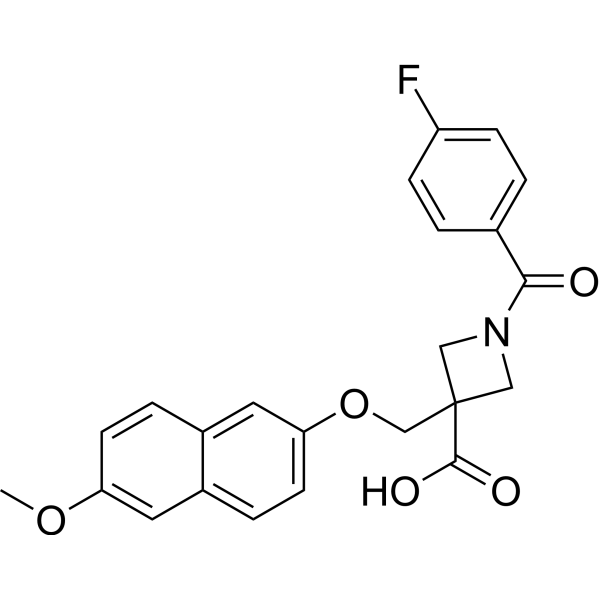
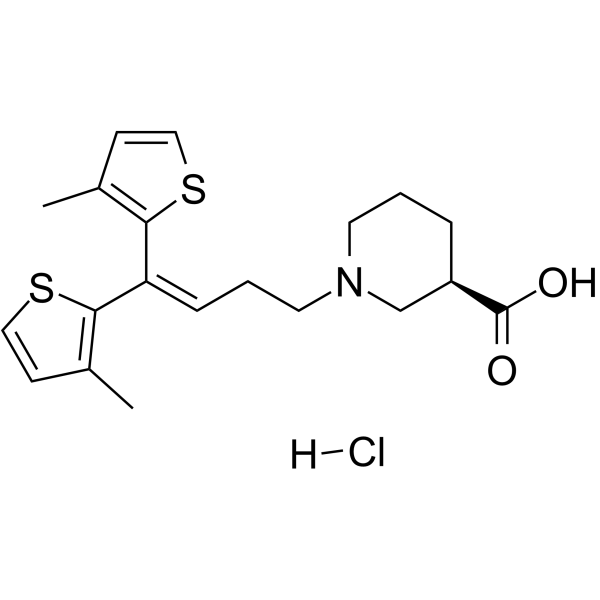
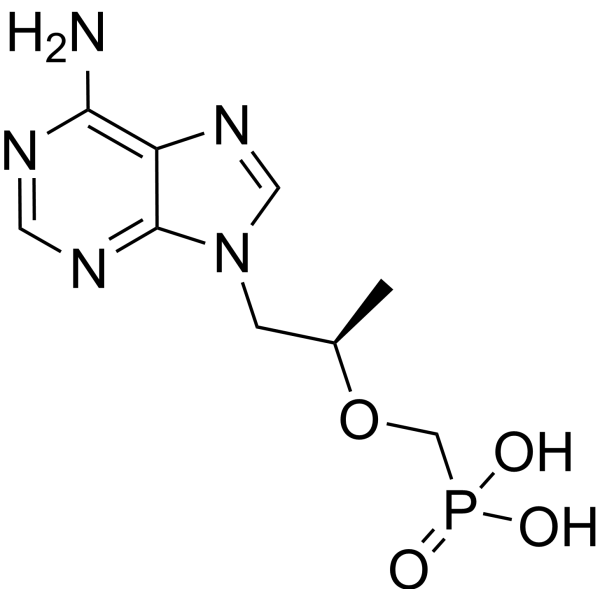
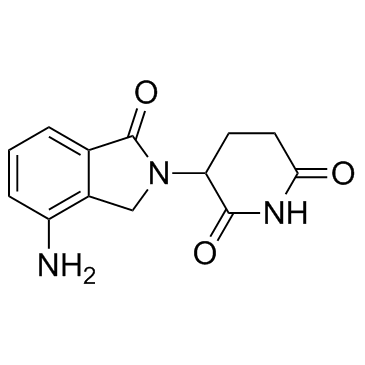
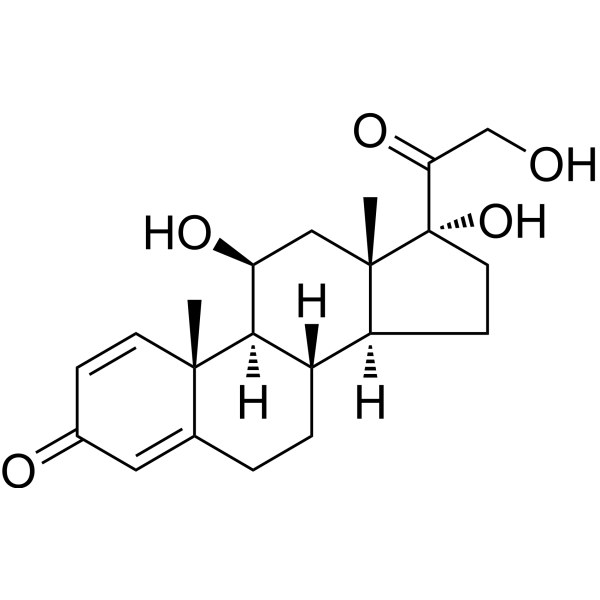
- Home
-
Signaling PathwaysAutophagy Angiogenesis ApoptosisMDM-2/p53Cell CycleTNF-alpha/TNF ReceptorBcl-2CaspaseFerroptosisRIP kinaseApoptosisThymidylate SynthaseIAPDNA/RNA SynthesisCytoskeletal Signaling DNA Damage/DNA RepairCDKAurora KinasePERKPPARAntifolateHSP (Heat shock proteins)TOPKChk inhibitorKinesinMicrotubule/TubulinMycCRISPR/Cas9Endocrinology & Hormones EpigeneticsTopoisomeraseDNA Alkylator/CrosslinkerTelomeraseHDACDNA/RNA SynthesisNucleoside Antimetabolite/AnalogDNA StainPARPATM/ATRSirtuinG-quadruplexCyclin G-associated Kinase (GAK)DNA-PKRAD51HDACGPCR & G ProteinEpigenetic Reader DomainHistone MethyltransferaseHistone AcetyltransferaseHistone DemethylaseDNA MethyltransferaseAMPKAdenylate CyclaseImmunology & InflammationAdenosine ReceptorProstaglandin ReceptorGCGR(Glucagon Receptor)mAChRLPL ReceptorMelatonin ReceptorAngiotensin ReceptorCXCROxytocin ReceptorCGRP ReceptorSomatostatin Receptor (SSTR)GNRH ReceptorGLP ReceptorRasHistamine ReceptorNeurokinin ReceptorEndothelin ReceptorP2Y ReceptorMelanocortin ReceptorOpioid ReceptorG protein-coupled Bile Acid Receptor 1Guanylate CyclaseBombesin ReceptorSecretin ReceptorCaSRCannabinoid ReceptorLeukotriene ReceptorG Protein-coupled Receptor Kinase (GRK)S1P ReceptorToll-like Receptor (TLR)MAPK/ERK Pathway Membrane Transporter/Ion ChannelNO SynthaseNeuraminidaseAryl Hydrocarbon Receptor (AhR)Complement SystemNOD-like Receptor (NLR)Reactive Oxygen SpeciesSTINGCOX(Cyclooxygenase)LAG-3Nuclear Factor of activated T Cells (NFAT)PD-1/PD-L1CTLA-4InterleukinsCD markersTim3MesothelinIFNARCD2CCRAP-1FAPNectin-4Thrombopoietin ReceptorMacrophage migration inhibitory factor (MIF)SODMucinSalt-inducible Kinase (SIK)IRAKPGE synthasePSMA(Prostate-specific membrane antigen)CD38NKCCMetabolismP-glycoproteinChloride ChannelPotassium ChannelCalcium ChannelATPaseSodium ChanneliGluRTRP ChannelMonoamine TransporternAChRSGLTATP SynthaseVDACProton PumpEAATCRM1Apical Sodium-Dependent Bile Acid Transporter;ASBTCFTRMonocarboxylate Transporter (MCT)RAR/Retinoid X Receptors (RXR)Microbiology/Virology/Anti-infectionHIF/HIF Prolyl-HydroxylaseDrug MetaboliteGlucosylceramide SynthaseCytochrome P450HydroxylaseCETPEndogenous MetaboliteFactor XaDehydrogenasePhosphataseHMG-CoA Reductase(HMGCR)Carbohydrate MetabolismGlucosidaseLipaseFXR11β-HSDPCSK9Factor VIIICarbonic AnhydraseLDLSer/Thr ProteaseFatty Acid Synthase (FASN)Mineralocorticoid ReceptorPhospholipaseAdiponectin ReceptorAcyltransferasePhosphodiesterase (PDE)COMTDopamine β-hydroxylaseFAAHAldose ReductaseOxidative PhosphorylationAngiotensin-converting Enzyme (ACE)LiposomePAI-15 alpha ReductaseCathepsinHydroxylase inhibitorLXRIsocitrate Dehydrogenase (IDH)Thyroid Hormone Receptor | THRE1/E2/E3 EnzymePyruvate KinaseReninCholesterol metabolism ATP Citrate LyaseHexokinaseAcetyl-CoA CarboxylaseReverse TranscriptaseNeuronal SignalingInfluenza VirusHIV ProteaseHCV ProteaseSARS-CoVIntegraseCMVAntibioticsBacterialParasiteRSVHBVFungalDengue virusβ-lactamase inhibitorAntiviralVirus ProteaseHSV5-HT ReceptorNF-κB PI3K/Akt/mTOR ProteasesGABA ReceptorDopamine ReceptorSerotonin TransporterAmyloid-βMonoamine OxidasemGluRCholinesterase(ChE)Adrenergic ReceptorNMDARMAOAChR ReceptorOrexin ReceptorGlyTSigma ReceptorCaMKCalcineurinAdenosine KinaseProteasomeProtein Tyrosine Kinase/RTKMMPHIV ProteaseTyrosinaseGlutaminaseAminopeptidaseXanthine OxidaseDipeptidyl Peptidase(DPP)ThrombinElastaseFarnesyl TransferaseEGFRStem Cells & Wnt TGF-beta/Smad Vitamin D Related Antibody-drug Conjugate/ADC Related PROTAC JAK/STAT Signaling Ox Stress ReagentsFGFRVEGFRFLT3Insulin ReceptorIGF-1RBcr-AblBtkEphrin receptorc-Fmsc-Met/HGFRPDGFRDYRKSrcROS KinaseAnaplastic lymphoma kinase (ALK)AxlSykCSF-1RRET(REarranged during Transfection)FAK (PTK2)Tie (Angiopoietin receptor)
-
ProductsSignaling PathwaysAutophagy Angiogenesis ApoptosisLife ScienceMDM-2/p53 TNF-alpha/TNF Receptor Bcl-2 Caspase Ferroptosis RIP kinase Apoptosis Thymidylate Synthase IAPCell CycleDNA/RNA Synthesis CDK Aurora Kinase PERK PPAR Antifolate HSP (Heat shock proteins) TOPK Chk inhibitor Kinesin Microtubule/Tubulin MycCytoskeletal Signaling DNA Damage/DNA RepairCRISPR/Cas9 Topoisomerase DNA Alkylator/Crosslinker Telomerase HDAC DNA/RNA Synthesis Nucleoside Antimetabolite/Analog DNA Stain PARP ATM/ATR Sirtuin G-quadruplex Cyclin G-associated Kinase (GAK) DNA-PK RAD51Endocrinology & Hormones EpigeneticsHDAC Epigenetic Reader Domain Histone Methyltransferase Histone Acetyltransferase Histone Demethylase DNA Methyltransferase AMPKGPCR & G ProteinAdenylate Cyclase Adenosine Receptor Prostaglandin Receptor GCGR(Glucagon Receptor) mAChR LPL Receptor Melatonin Receptor Angiotensin Receptor CXCR Oxytocin Receptor CGRP Receptor Somatostatin Receptor (SSTR) GNRH Receptor GLP Receptor Ras Histamine Receptor Neurokinin Receptor Endothelin Receptor P2Y Receptor Melanocortin Receptor Opioid Receptor G protein-coupled Bile Acid Receptor 1 Guanylate Cyclase Bombesin Receptor Secretin Receptor CaSR Cannabinoid Receptor Leukotriene Receptor G Protein-coupled Receptor Kinase (GRK) S1P ReceptorImmunology & InflammationToll-like Receptor (TLR) NO Synthase Neuraminidase Aryl Hydrocarbon Receptor (AhR) Complement System NOD-like Receptor (NLR) Reactive Oxygen Species STING COX(Cyclooxygenase) LAG-3 Nuclear Factor of activated T Cells (NFAT) PD-1/PD-L1 CTLA-4 Interleukins CD markers Tim3 Mesothelin IFNAR CD2 CCR AP-1 FAP Nectin-4 Thrombopoietin Receptor Macrophage migration inhibitory factor (MIF) SOD Mucin Salt-inducible Kinase (SIK) IRAK PGE synthase PSMA(Prostate-specific membrane antigen) CD38MAPK/ERK Pathway Membrane Transporter/Ion ChannelNKCC P-glycoprotein Chloride Channel Potassium Channel Calcium Channel ATPase Sodium Channel iGluR TRP Channel Monoamine Transporter nAChR SGLT ATP Synthase VDAC Proton Pump EAAT CRM1 Apical Sodium-Dependent Bile Acid Transporter;ASBT CFTR Monocarboxylate Transporter (MCT)MetabolismRAR/Retinoid X Receptors (RXR) HIF/HIF Prolyl-Hydroxylase Drug Metabolite Glucosylceramide Synthase Cytochrome P450 Hydroxylase CETP Endogenous Metabolite Factor Xa Dehydrogenase Phosphatase HMG-CoA Reductase(HMGCR) Carbohydrate Metabolism Glucosidase Lipase FXR 11β-HSD PCSK9 Factor VIII Carbonic Anhydrase LDL Ser/Thr Protease Fatty Acid Synthase (FASN) Mineralocorticoid Receptor Phospholipase Adiponectin Receptor Acyltransferase Phosphodiesterase (PDE) COMT Dopamine β-hydroxylase FAAH Aldose Reductase Oxidative Phosphorylation Angiotensin-converting Enzyme (ACE) Liposome PAI-1 5 alpha Reductase Cathepsin Hydroxylase inhibitor LXR Isocitrate Dehydrogenase (IDH) Thyroid Hormone Receptor | THR E1/E2/E3 Enzyme Pyruvate Kinase Renin Cholesterol metabolism ATP Citrate Lyase Hexokinase Acetyl-CoA CarboxylaseMicrobiology/Virology/Anti-infectionReverse Transcriptase Influenza Virus HIV Protease HCV Protease SARS-CoV Integrase CMV Antibiotics Bacterial Parasite RSV HBV Fungal Dengue virus β-lactamase inhibitor Antiviral Virus Protease HSVNeuronal Signaling5-HT Receptor GABA Receptor Dopamine Receptor Serotonin Transporter Amyloid-β Monoamine Oxidase mGluR Cholinesterase(ChE) Adrenergic Receptor NMDAR MAO AChR Receptor Orexin Receptor GlyT Sigma Receptor CaMK Calcineurin Adenosine KinaseNF-κB PI3K/Akt/mTOR ProteasesProteasome MMP HIV Protease Tyrosinase Glutaminase Aminopeptidase Xanthine Oxidase Dipeptidyl Peptidase(DPP) Thrombin Elastase Farnesyl TransferaseProtein Tyrosine Kinase/RTKEGFR FGFR VEGFR FLT3 Insulin Receptor IGF-1R Bcr-Abl Btk Ephrin receptor c-Fms c-Met/HGFR PDGFR DYRK Src ROS Kinase Anaplastic lymphoma kinase (ALK) Axl Syk CSF-1R RET(REarranged during Transfection) FAK (PTK2) Tie (Angiopoietin receptor)Stem Cells & Wnt TGF-beta/Smad Vitamin D Related Antibody-drug Conjugate/ADC Related PROTAC JAK/STAT Signaling Ox Stress ReagentsMolecular BiologyChemistryPharma Development & Drug Discovery ResearchCell BiologyBiochemicals & Reagents Glycoscience (Carbohydrates) Impurities & Metabolites Amino Acids and DerivativesFmoc-amino acids Boc-amino acids Z-amino acids Unnatural amino acids Amino alcohols N-methyl amino acids Serine and derivatives Alanine and derivativesPolypeptide and Protein EnzymeBio-organic Synthesis Catalysts and Ligands Building BlocksAnalytical Chemistry Materials Science Natural ProductsAliphatic heterocycle compounds Aromatic heterocycle compounds Aromatic ring compounds Alkynes Fatty chain compounds Bridge Rings compounds Amine Inorganic acid ester Fatty ring compounds Ketone compoundsStable IsotopesPharmaceutical IntermediateOxidation reagentAntioxidantIsoprenoids Nitrogen-containing Compounds Phenylpropanoids FlavonoidsInhibitory Antibodies Research AreasFlavones/Flavanones Flavanes/Isoflavans/Flavanols Flavonols/Flavanonols Isoflavones/Isoflavanones Chalcones/DihydrochalconesQuinonesPhenolic Compounds CarbohydratesChalconesAliphatic CompoundsBotanical Extracts
Search history
No search history
Delete history